INTRODUCING
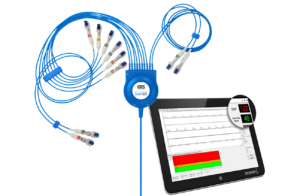
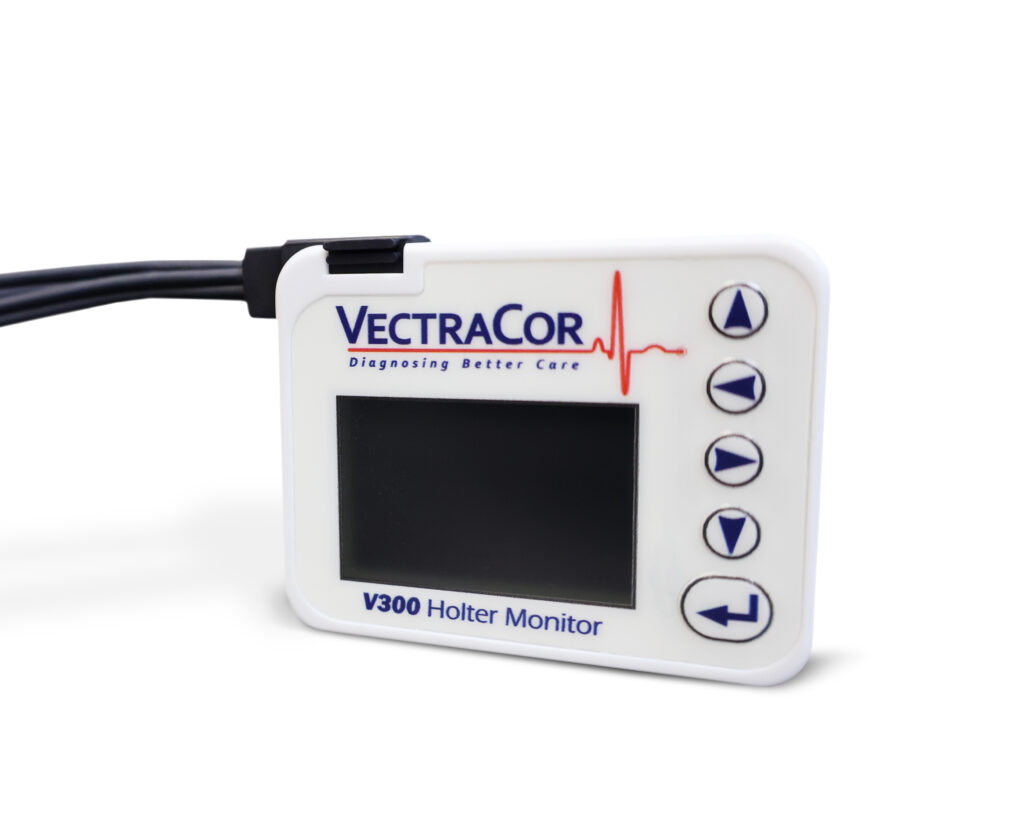
THE V300 HOLTER
Our new Holter hardware and software is the perfect addition to your practice. The easy-to-use software will help make your workflow more efficient!
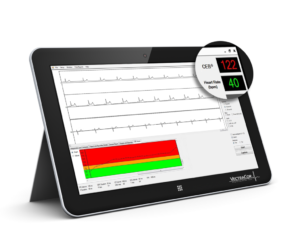
Cardiac Electrical Biomarker (CEB®)
Detects ECG changes suggestive of a heart attack.
Email - Data Transfer Capability
Allows you to email an ECG report to anyone.
EMR Connectivity
We connect to most major EMR systems.
What we do?
VectraCor uses its patented technology to design and develop medical devices to simplify and reduce costs of diagnostic testing as well as improve patient care. Our latest technology, VectraplexECG can detect ECG changes suggestive of a heart attack and derive a 15-22 lead ECG utilizing only 5-electrodes. We have a entire suite of products that are durable, mobile, and ready to turn an off-the-shelf tablet or laptop into a medical device. Our products are FDA cleared, CE marked, and VectraCor is ISO-13485 certified.
Heart Disease Stroke and Research Statisitcs At-a-Glance
by American Heart Association
People in the U.S have heart attacks each year.
1
+
Have a heart attack for the first time each year.
1
+
People die each year from a heart attack.
1
+
Have recurrent heart attacks.
1
+